Allegheny Health Network’s media relations team is dedicated to providing reporters and other members of the news media with the assistance they need.
AHN team performs region’s first deep brain stimulation therapy to treat opioid addiction
PITTSBURGH – A multidisciplinary team of physicians at Allegheny Health Network (AHN) is among the first in the nation to safely investigate the use of deep brain stimulation therapy (DBS) to treat a patient suffering from opioid addiction.
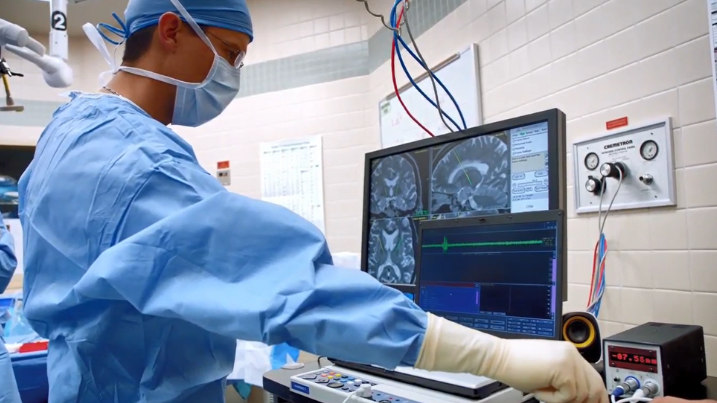
Led by principal investigator and AHN neurosurgeon Nestor D. Tomycz, MD, the AHN team announced this week that they have successfully implanted an deep brain stimulation (DBS) device in the brain of a 28-year-old New York man with treatment-resistant opioid addiction.
The procedure took place in December at AHN’s Allegheny General Hospital, and the patient’s ongoing therapy is part of an FDA-approved clinical study.
DBS for opioid-use disorder involves implanting bilateral electrode leads into an area of the brain called the nucleus accumbens – the brain’s addiction and reward center. The electrodes are powered by a pacemaker-like device implanted near the patient's collarbone. By targeting this area of the brain with continuous electrical pulses, the goal is to reduce the severe cravings that lead to frequent relapse and overdose in patients addicted to opioids.
“Drug addiction, particularly to opioids, is a devastating brain disease that causes more deaths in America than car accidents, and there is an urgent need for new treatments,” said Dr. Tomycz. "AHN is among the first health care organizations in the nation to study DBS for opioid addiction, and we are excited to be a part of this groundbreaking effort.”
Opioid misuse remains one of the nation’s leading public health problems. According to the Centers for Disease Control and Prevention, in 2023, drug overdoses killed more than 110,000 people in the U.S., and most of those were related to opioids, especially powerful synthetic opioids such as fentanyl.
DBS has been around since the 1980s and has become a well-established treatment for movement disorders such as Parkinson’s disease and essential tremors. Under the leadership of Donald Whiting, MD, AHN's Chief Medical Officer and chair of the AHN Neuroscience Institute, AGH has become a top national center for DBS treatment and research.
“DBS in itself is not a new concept, but we are proud to be one of the few centers in the country where this innovative technology is being studied for new indications, including obesity and addiction,” said Dr. Whiting.
The AHN study was approved by the U.S. Food and Drug Administration and AHN’s Institutional Review Board (IRB), and it is being funded by Abbott.
“COVID worsened the opioid crises in the United States and continues to cause a staggering number of deaths among young Americans,” said Elizabeth Cuevas, MD, division chief of AHN’s Center for Inclusion Health, which houses AHN’s addiction medicine programs. “Addiction is a brain disease – it’s not a bad habit that people can just stop. Those who have an opioid addiction have had their brain hijacked by drugs.”
Following the implantation of the device, patients enrolled in the AHN study will be closely monitored for two years by the multidisciplinary research team. A total of three patients can be enrolled in the ongoing trial.
“Our first patient suffered nearly 15 opioid overdoses despite intensive medical and rehabilitative treatments,” said Dr. Tomycz. “He traveled from New York to Pittsburgh for help after all other treatments failed. I’m pleased to announce his recent surgery was a success, and now our team will monitor his recovery.”
To qualify for AHN's trial, eligible patients must have opioids such as heroin and fentanyl as their primary drug of addiction, and they must have failed multiple treatments for addiction. Those interested in learning more about AHN's study of DBS for opioid use disorder can contact neuroscience research manager Sarah Kimutis at 412-359-3565.

